December 2, 2020 – Abbott (Abbott Park, IL) announced that its next-generation, sensor-based glucose monitoring technology, FreeStyle Libre 2, received approval by Health Canada for adults and children (age 4 and older) with diabetes.
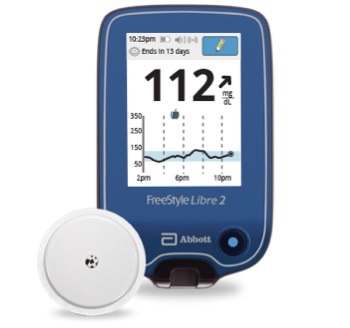
With new features such as optional, real-time alarms that measure glucose levels every minute, FreeStyle Libre 2 gives users the option to be alerted in real-time of critical events such as hypoglycemia (low glucose levels) or hyperglycemia (high glucose levels).
The wearable technology eliminates the need for fingersticks and provides excellent accuracy and actionable information to help people with diabetes better manage their condition, Abbott says.
The device will be priced at the same cost as the current FreeStyle Libre system.
Using Bluetooth technology, the FreeStyle Libre 2 system automatically alerts users when their glucose is high or low without needing to scan the sensor.
The FreeStyle Libre 2 sensor is worn on the back of the upper arm for up to 14 days and measures glucose every minute to help users and their healthcare providers make informed treatment decisions.
With a one-second scan using FreeStyle LibreLink, a smartphone app, or handheld reader, users can see their glucose reading, trend arrow and eight-hour history. Users can also share data with their physicians or family members via the LibreLinkUp mobile app.
