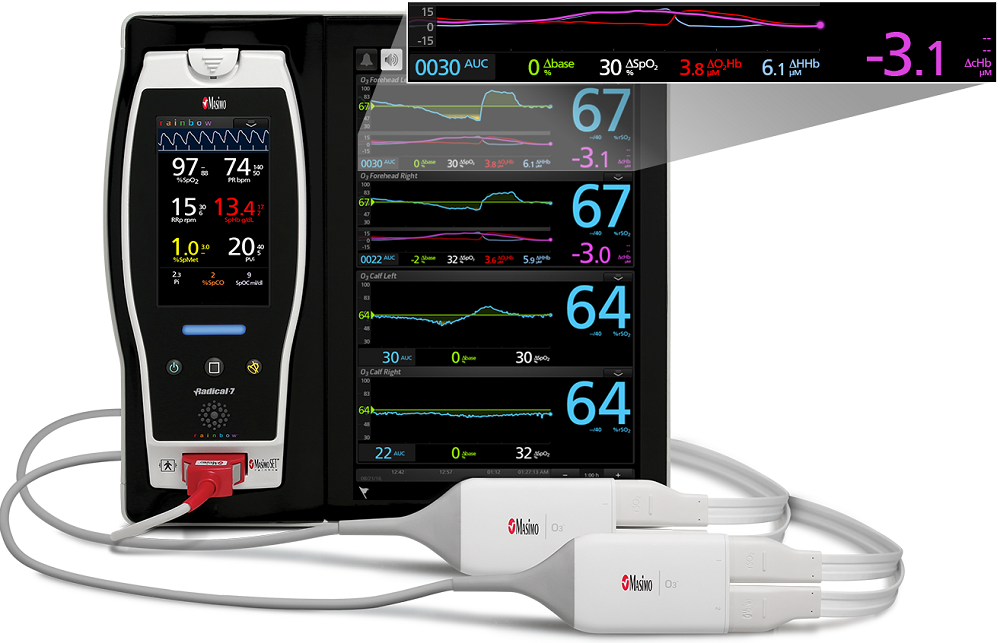
September 1, 2020 – Masimo (Irvine, CA) announced that O3 Regional Oximetry has received FDA clearance for expanded use in monitoring somatic tissue oxygenation saturation in all patient populations and monitoring relative changes in hemoglobin, oxyhemoglobin, and deoxyhemoglobin in adult brains.
This clearance means that O3 is now indicated for use in both cerebral and somatic applications, both in the U.S. and in CE-mark countries, for all patient populations.
O3 seamlessly integrates with Masimo SedLine brain function monitoring on the Root Patient Monitoring and Connectivity Platform, a powerful, expandable hub that integrates an array of technologies, devices, and systems to provide multimodal monitoring and connectivity solutions. Root’s plug-and-play expansion capabilities allow clinicians to simultaneously monitor with O3, SedLine, and other measurements, such as SET Measure-through Motion and Low Perfusion pulse oximetry, providing clinicians with expanded visibility of oxygenation status. Additional modalities available on Root include advanced rainbow noninvasive measurements such as total hemoglobin (SpHb) and PVi (an indicator of fluid responsiveness), NomoLine capnography, and more – all via an easy-to-interpret, customizable display. Using Root in combination with Masimo Iris Gateway, monitoring data from O3 can be automatically charted in electronic medical records (EMRs).