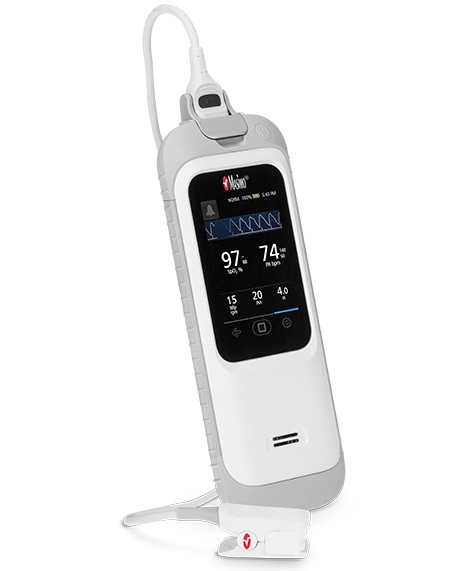
September 29, 2020 – Masimo (Irvine, CA) received FDA clearance of the Rad-G Pulse Oximeter, a handheld device that provides SET pulse oximetry, respiration rate from the pleth (RRp), and other vital parameters for both spot-checking and continuous monitoring.
With its long-lasting rechargeable battery, robust rubber casing, light weight, and a convenient new direct-connect sensor capable of monitoring both adults and children, Rad-G makes it easier for clinicians to quickly assess patients and make informed care decisions anywhere pulse oximetry or vital signs checking is needed in a compact, portable form factor, the company says.
Rad-G can be used in a variety of settings, including but not limited to physicians’ offices, outpatient services, long-term care facilities, wellness clinics, first-response scenarios, and limited-resource environments.
Launching alongside the device, the new multipurpose, direct-connect Rad-G Sensor is indicated for monitoring both adult and pediatric patients. By eliminating the need to stock and carry multiple sensor types, the Rad-G Sensor further increases Rad-G’s versatility and ease of use, especially in more challenging field environments.
In addition to the new sensor, Rad-G is compatible with the vast portfolio of Masimo reusable and single-patient-use sensors, maximizing its flexibility and offering clinicians the ability to customize the solution based on the unique needs of each care setting.
