January 15, 2021 – Medtronic plc announced the first enrollment in ADAPT-PD (Adaptive DBS Algorithm for Personalized Therapy in Parkinson’s Disease), its trial evaluating the safety and efficacy of adaptive deep brain stimulation (aDBS) in patients with Parkinson’s Disease (PD).
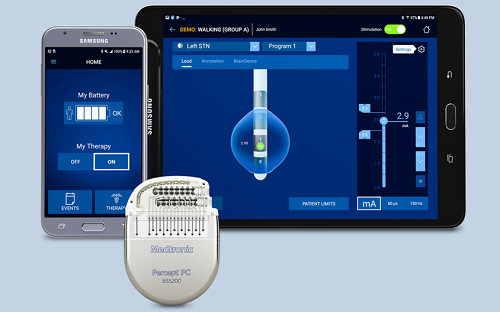
Adaptive deep brain stimulation is an investigational feature of the Percept PC device that could be enabled if approved. The investigational feature used in this study allows for automated adjustment of brain stimulation to provide therapy to manage symptoms of Parkinson’s disease based on a patient’s clinical state, the company said.
The randomized study(opens new window) will take place across 12 study sites at leading Movement Disorders research centers in the United States, Europe, and Canada. An estimated 36 subjects will undergo a total evaluation period of 15 months.
While the aDBS feature is investigational and has not been approved for commercial use, the Percept PC device (cDBS) was approved by the FDA in June 2020. The Percept PC device (cDBS) utilizes proprietary BrainSense technology making it the only DBS system with the ability to capture patient-specific brain signals.
The sensing feature of the Percept PC system is intended for use in patients receiving DBS where chronically-recorded bioelectric data may provide useful, objective information regarding patient clinical status. Clinical benefits of brain sensing have not been established.
To date, more than 175,000 patients have been implanted with Medtronic DBS devices for management of Parkinson’s symptoms and other conditions such as Essential Tremor, Epilepsy, Dystonia and OCD.
