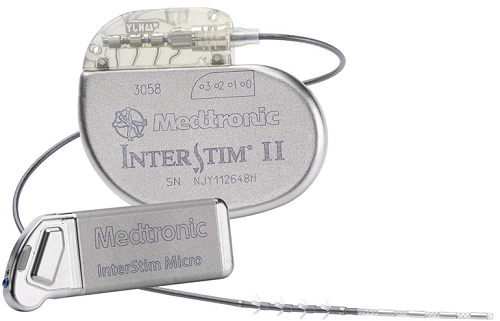
February 18, 2021 – Medtronic plc (Dublin, Ireland) has received approval from the United States Food and Drug Administration (FDA) for expanded MRI labeling of its InterStim II and InterStim Micro sacral neuromodulation (SNM) systems that use SureScan MRI leads.
The updated MRI Guidelines allow for a wider range of MRI scan parameters and shorter wait time between MRI scans, thereby improving patient access to MRI exams and adding flexibility for MRI providers.
It applies to existing and future implants of InterStim systems that use SureScan MRI leads.
The new Medtronic scanning parameters1 increases SAR limits for 1.5 Tesla scans from 0.5 W/kg to 2.0 W/kg; and for 3 Tesla scans from 0.5 W/kg to 1.4 W/kg.
The new labeling also decreases wait time from 60 minutes to 5 minutes for maximum duration scans (30 minutes).
These guideline updates are in addition to the benefit already provided by SureScan MRI technology that impedance checks prior to an MRI scan are not required, providing more efficiency in patient care versus the other SNM system on the market, the company says.
