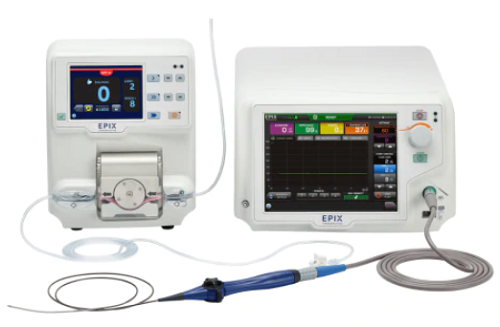
January 29, 2021 – Medtronic plc has received U.S. Food and Drug Administration (FDA) approval of the DiamondTemp Ablation (DTA) system which treats patients with recurrent, symptomatic paroxysmal atrial fibrillation (AF) and who have been unresponsive to drug therapy.
The DiamondTemp system is the first FDA-approved, temperature-controlled, irrigated radiofrequency (RF) ablation system with diamonds currently available to deliver ablations, the company says.
The DTA system is a temperature-controlled open-irrigated RF ablation system designed to deliver RF energy (heat) during ablations. The DTA system delivers real-time feedback on physical parameters, which can guide physicians as the lesion is being produced.
The DTA catheter is embedded with industrial-grade diamonds, which have 200-400 times greater thermal conductivity when compared to materials used in conventional RF ablation catheters. This thermal conductivity enables a low irrigation flow-rate, and accurate real-time measurements of tissue temperature, resulting in more efficient energy delivery. As the only open-irrigated RF catheter with industrial diamonds to optimize power based on tissue temperature, the DTA system is safe and effective and has demonstrated procedural efficiencies when compared to conventional contact force-sensing RF catheters.
Additionally, the DTA catheter is specially designed for recording of high-resolution electrogram (EGM) signals. Physicians can use these clear EGM signals as a physical indicator of lesion formation, as well as guidance for ablation location.
