August 20, 2020 – Masimo announced that, in a study recently published in the Journal of Tropical Pediatrics, researchers evaluating Masimo SpHb (noninvasive, continuous hemoglobin monitoring) on neonatal patients concluded that the technology “offers reliable Hb [hemoglobin] values, which are comparable with the more traditional tHb [invasive venous blood sampling].”
Noting that “iatrogenic anemia caused by diagnostic blood sampling is a common problem in intensive care units,” especially in neonatal intensive care units (NICUs), and that diagnostic blood sampling also has implications for infection control and health care costs, Drs. Halil Kazanasmaz and Mahmut Demir at Harran University in Sanliurfa, Turkey sought to assess the efficacy of noninvasive, continuous hemoglobin monitoring with SpHb on neonates by comparing it to conventional tHb.
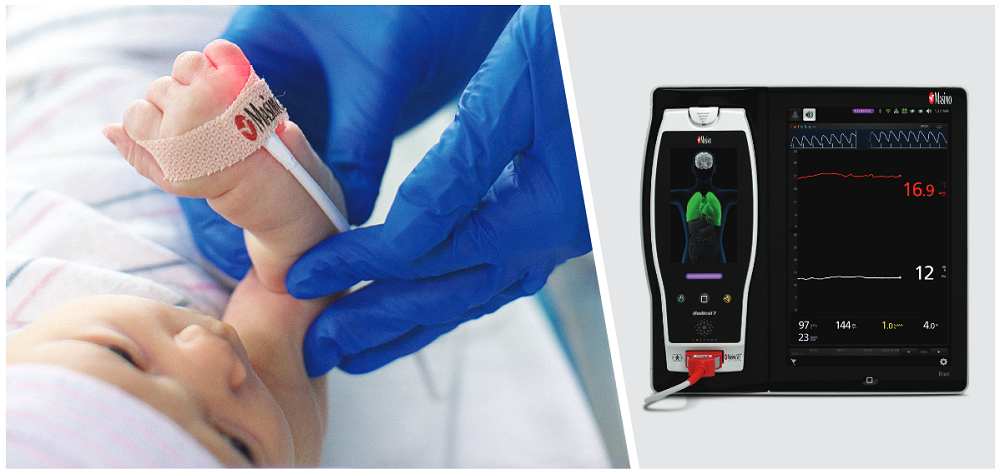
To investigate this, they studied 310 neonatal patients in a level III NICU in Turkey. Immediately after patients had blood drawn for tHb analysis using a hematological laboratory analyzer, their SpHb values (alongside other noninvasive measurements) were recorded using a Masimo Radical-7 Pulse CO-Oximeter with rainbow sensors placed on the neonate’s left foot.
SpHb is not intended to replace laboratory blood testing. Clinical decisions regarding red blood cell transfusions should be based on the clinician’s judgment considering, among other factors, patient condition, continuous SpHb monitoring, and laboratory diagnostic tests using blood samples.
Noninvasive, continuous SpHb for neonatal patients received CE marking in August 2019, and SpHb is now available for patients of all ages in CE marked countries. Noninvasive, continuous SpHb has received FDA clearance for patients > 3 kg but is not currently indicated for patients < 3 kg in the U.S.