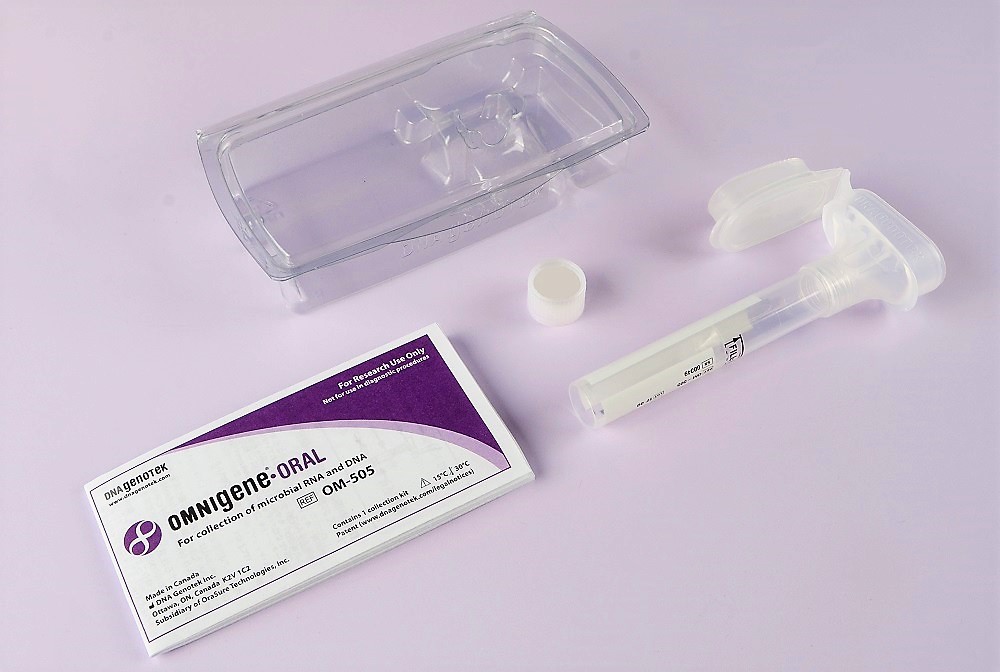
October 20, 2020 – OraSure Technologies, Inc. (Bethlehem, PA) announced its DNA Genotek subsidiary has received Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA) for the use of DNA Genotek’s OMNIgene·ORAL (OM-505, OME-505) saliva collection and stabilization device in COVID-19 testing.
This is the first FDA EUA that allows for the unsupervised use of the device at-home or in a healthcare setting when used as part of an approved or validated at-home test kit, meaning patients can safely collect their own sample, without the presence of a healthcare professional.
With this FDA authorization, OMNIgene·ORAL devices can be used for the self-collection, transport and laboratory testing of saliva specimens suspected of containing SARS-CoV-2 ribonucleic acid (RNA). This EUA follows the CE marking of OMNIgene·ORAL (OME-505) for in vitro diagnostic use, including for COVID-19 testing, in the EU.
The DNA Genotek kits for detection of the SARS-CoV-2 virus have been included in six customer EUAs. The OMNIgene·ORAL device has been included in EUAs granted to Clinical Reference Laboratory (CRL) and P23. The OraCollect·RNA device has been included in EUAs granted to Biocerna LLC, MiraDx and Quadrant Biosciences. The Oragene·Dx device has been included in an EUA granted to Phosphorus Diagnostics.
DNA Genotek’s devices support both PCR and sequencing-based COVID-19 tests, and its collection kits can be used for collection at home by consumers or by laboratory staff and healthcare providers.
