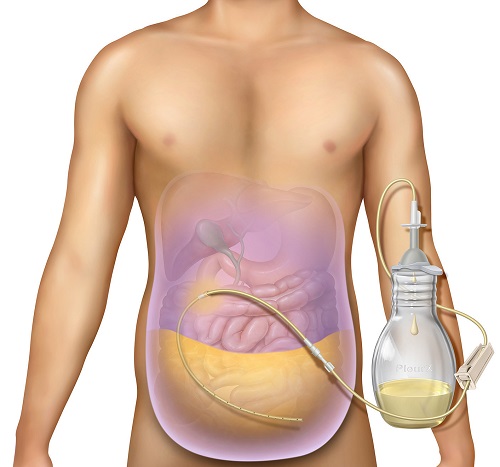
June 23, 2021 – BD (Becton, Dickinson and Company) (Franklin Lakes, NJ) announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the PeritX Peritoneal Catheter System for the drainage of symptomatic, recurrent non-malignant ascites.
The PleurX Peritoneal Catheter System was introduced in 2005 for the drainage of malignant ascites.
With the new, expanded indication, the system is being rebranded as the PeritX Peritoneal Catheter System to make it easier for physicians to navigate BD’s portfolio of at-home drainage products.
The PeritX Peritoneal Catheter System is the first and only FDA indicated tunneled catheter for the drainage of malignant and non-malignant ascites and for the palliation of symptoms related to recurrent ascites, a debilitating condition that causes the build-up of fluid in the abdomen.
BD says the condition can make ordinary daily activities more challenging. The fluid may also move to the chest and surround the lungs, making breathing more difficult.
