September 23, 2021 – OraSure Technologies, Inc. (Bethlehem, PA) announced that the Biomedical Advanced Research Development Authority (BARDA), part of the office of the Assistant Secretary for Preparedness and Response at HHS, will provide up to $13.6 million in funding for the company to obtain 510(k) clearance and Clinical Laboratory Improvement Amendments (CLIA) waiver for OraSure’s InteliSwab COVID-19 rapid test from the FDA.
Following 510(k) clearance, the company will pursue Clinical Laboratory Improvement Amendments (CLIA) waiver for InteliSwab, ensuring the test can continue to be performed by an untrained user outside of the laboratory setting.
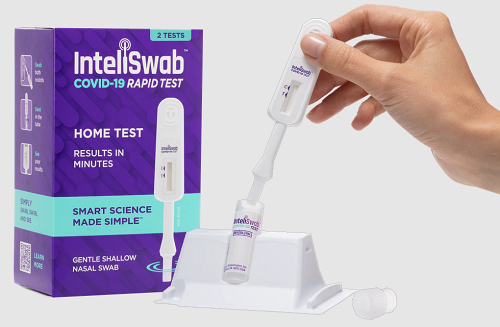
InteliSwab is a “swab, swirl, and see” test that uses an integrated swab to self-collect a sample from the lower nostrils. The result appears right on the test stick within 30 minutes, with no instruments, batteries, smartphone or laboratory analysis needed.
The product currently has three Emergency Use Authorizations (EUAs) from the FDA for professional point-of-care use, prescription (Rx) home use, and over-the-counter (OTC) use.
The InteliSwab COVID-19 Rapid Test is authorized for emergency use for the duration of the public health declaration. With FDA 510(k) clearance, InteliSwab can continue to be marketed without a public health emergency declaration from the Secretary of the Department of Health and Human Services (HHS).
