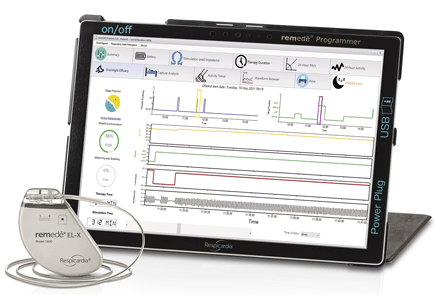
August 5, 2021 – ZOLL Medical Corporation (Chelmsford, MA), an Asahi Kasei company, announced it has received U.S. Food and Drug Administration (FDA) approval for the remedē EL-X System to treat moderate to severe Central Sleep Apnea (CSA) in adult patients.
The next-generation remedē EL-X System combines enhanced functionality with a patient-friendly design, simplifying the implant procedure and providing greater device longevity for patients with Central Sleep Apnea.
Features of the new device include:
- Extended longevity: 40% longer average battery life versus previous version
- Reduced size: Approximately 25% smaller than the previous version
- Simplified implant: Single lead, single-port system that provides both stimulation and sensing from a single lead
- Data-driven clinical insights with DRēAM View: Enhancements include full-night, comprehensive diagnostic capabilities
A phased launch of the remedē EL-X System will begin immediately in implanting centers in the U.S.
