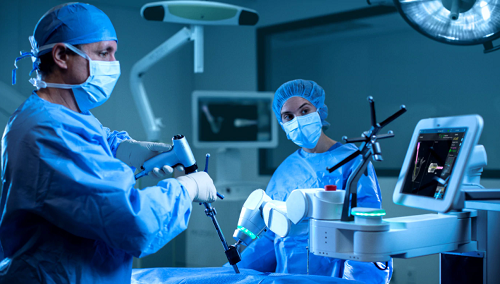
December 16, 2020 – Medtronic plc (Dublin, Ireland) announced that the U.S. Food and Drug Administration (FDA) cleared the use of navigated interbody and Midas Rex high speed drills with the Mazor Robotic Guidance System earlier than originally anticipated.
The Mazor platform now provides surgeons with unprecedented procedural integration by seamlessly combining the power of Midas Rex drills with the market leading visibility and navigation from the StealthStation software.
According to the company, the solution delivers the confidence of access and interbody navigation and predictability of planning, along with the precision of robotic technology that surgeons expect from the Mazor Robotic Guidance System and the exclusive Medtronic robotics portfolio.
Medtronic has evolved the Mazor platform to allow surgeons to quickly visualize anatomy and spinal structures in relation to one another in 3D. The enhanced interface delivers fast and seamless access to plan and simulate cages and screws, with the goal of increasing efficiencies for surgeons.
The Midas Rex High Speed Drill Systems are now fully integrated throughout the Mazor procedure enabling improved trajectory precision starting with pilot hole creation and offering attachments and dissecting tools designed for accurate drilling with speeds up to 75,000 rpm. Additionally, surgeons can now utilize navigated interbody features on the Mazor system to visualize disc prep and interbody placement during a robotic procedure.
